Quality Policy
Our mission at Sparsha Pharma is to develop clinically effective, innovative and affordable Transdermal therapeutic products to improve the quality of life of patients in need of such therapies.
Sparsha Pharma products are to meet international standards of quality.
We, as a Team, dedicate ourselves to achieve this objective by maintaining quality every stage and by complying with all current regulatory requirements.

Manufacturing Facilities
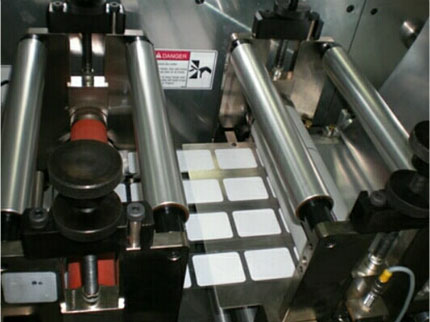
Sparsha Pharma's manufacturing equipment enables the production of various types of patches. Quality control is capable of analyzing pharmaceutical raw materials as well as finished transdermal products.
Sparsha Pharma is open to discuss contract manufacturing service globally, and is willing to provide its capabilities for contract manufacturing of various transdermal patches. Sparsha Pharma has 20,000 sq. ft. of space reserved for GMP production facilities with space available for future growth. The production facility currently has 14,000 sq. ft. of production area and 3,000 sq. ft. of Quality Control laboratory.