Product Specification
| PRODUCT | STATUS |
|---|---|
| Fen-Touch | On market in India |
| Artho-Touch | On market in India |
| Diclo-Touch | On market in India |
| Tulobuterol Patch | In process of regulatory review |
Product Description
Chronic obstructive pulmonary disease (COPD) could even be a typical airway disorder. Especially, acute exacerbations of COPD (AECOPD) can significantly reduce pulmonary function. The bulk of AECOPD episodes are attributed to infections, although environmental stress also plays a task. Increasing today's urbanization and associated pollution, especially in developing countries, are shown to contribute to COPD pathogenesis. Elevated levels of particulate (PM) in polluted air are strongly correlated with the onset and development of various respiratory diseases. Reducing COPD PM2.5 levels could even be a viable approach to lower AECOPD incidence, attenuate COPD progression and reduce the associated healthcare burden.
Sparsha Pharma International Pvt. Ltd. was established in 2008 as the first transdermal company in India to engage in R&D, manufacturing and marketing of transdermal products for global markets. We offer COPD pm2.5 drug medicine by introducing patches and we are the India's first Who Certified transdermal patches company.

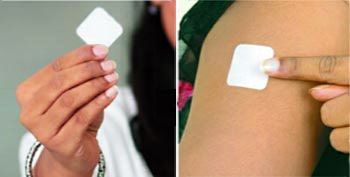
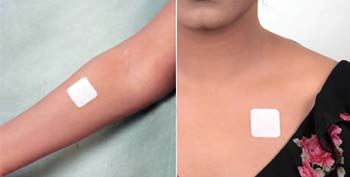
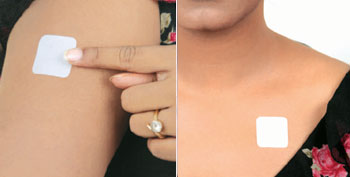
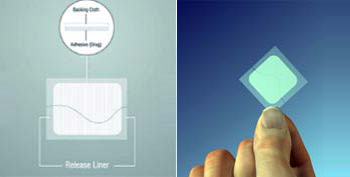
How to Apply
- Tear open the pouch from the opening notch
- Remove the Fen-Touch from the Pouch, just before applying it on the skin
- Peel away one side of the release liner, then place the patch on the skin
- Remove the remaining side of the release liner and press the patch for 30 seconds with hand to ensure adhesiveness
Precautions:
- Apply patch immediately after taking out from the pouch
- Close the pouch after removal of the patch
- Protect from light
- Keep out of reach of children
- Apply patch to non hairy part on the body
- Apply patch once a day